Advanced Energy Storage Battery Technologies
Pioneering innovations in next-generation secondary batteries for sustainable energy systems, including breakthroughs that revolutionize solar energy battery storage capabilities.
The Future of Energy Storage is Here
As the global demand for efficient, sustainable energy solutions continues to rise, the development of advanced battery technologies has become paramount. From renewable integration to grid stabilization, these innovations are transforming how we store and utilize energy. Among their most critical applications is enhancing solar energy battery storage systems, making renewable energy more reliable and accessible than ever before.
High Energy Density
Next-generation batteries offer significantly higher energy storage capacity compared to traditional technologies.
Sustainability
Constructed using abundant materials with lower environmental impact throughout their lifecycle.
Long Cycle Life
Extended durability with thousands of charge-discharge cycles, reducing replacement frequency.
Enhanced Safety
Improved chemical stability reduces risks of thermal runaway and other safety concerns.
Secondary Magnesium-Based Battery Technology
Secondary magnesium-based battery technology represents one of the most promising alternatives to conventional lithium-ion batteries, offering significant advantages in terms of energy density, safety, and resource availability. Magnesium, being the eighth most abundant element in the Earth's crust, provides a sustainable foundation for large-scale energy storage solutions, particularly in solar energy battery storage applications where material abundance is crucial for widespread adoption.
The fundamental principle behind magnesium-ion batteries involves the reversible insertion/extraction of magnesium ions between the positive and negative electrodes during charge and discharge cycles. Unlike lithium, magnesium ions carry a +2 charge, enabling higher energy transfer per ion and contributing to the technology's impressive energy density potential, which can exceed 400 Wh/kg in laboratory settings.
One of the key advantages of secondary magnesium-based battery technology is its inherent safety. Magnesium does not form dendrites during cycling, the needle-like structures that can cause short circuits in lithium-ion batteries. This characteristic eliminates a major safety hazard and contributes to longer cycle life, with some prototypes demonstrating over 2,000 charge-discharge cycles with minimal capacity degradation.
Recent advancements in electrolyte development have addressed one of the primary challenges facing magnesium batteries. Early electrolytes suffered from poor conductivity and corrosion issues, but new formulations using organohaloaluminate complexes and other novel chemistries have significantly improved performance. These electrolytes not only enhance ion mobility but also enable operation across a wider temperature range, making magnesium batteries suitable for diverse environments from desert solar energy battery storage installations to cold-climate applications.
Cathode materials have also seen substantial innovation. Researchers have developed various transition metal oxides, sulfides, and selenides that can accommodate magnesium ions. Among the most promising are Chevrel phases (Mo₆S₈), which exhibit excellent stability and reversible magnesium insertion properties. Other candidates include layered oxides and polyanionic compounds, each offering unique benefits in terms of energy density, rate capability, and stability.
In terms of commercialization, several companies and research institutions are making significant strides. Toyota has announced plans to develop magnesium-ion batteries for automotive applications, while academic research groups at institutions like MIT and Stanford continue to push the boundaries of performance. The technology's potential in solar energy battery storage systems is particularly compelling, as its high energy density and long cycle life can effectively address the intermittent nature of solar power, providing reliable energy even during extended periods of low sunlight.
Despite these advancements, challenges remain. The relatively slow diffusion of magnesium ions in solid materials can limit charge-discharge rates, though nanostructuring electrode materials has shown promise in mitigating this issue. Additionally, scaling production processes to meet industrial demands while maintaining quality and cost-effectiveness presents ongoing engineering challenges.
Looking forward, secondary magnesium-based battery technology is poised to play a significant role in the energy transition. Its combination of high energy density, inherent safety, and material abundance positions it as a strong candidate for both mobile and stationary energy storage applications. As research continues to overcome remaining technical hurdles, we can expect to see magnesium batteries integrated into everything from electric vehicles to grid-scale solar energy battery storage systems, contributing to a more sustainable and resilient energy future.
Magnesium Battery Architecture
Cross-sectional diagram illustrating the key components of a modern magnesium-ion battery, including the magnesium metal anode, electrolyte, and Chevrel phase cathode.
Performance Metrics
Secondary Aluminum-Based Battery Technology
Secondary aluminum-based battery technology has emerged as a highly promising candidate in the field of energy storage, offering unique advantages that address many limitations of current battery systems. Aluminum's exceptional abundance—making up approximately 8% of the Earth's crust—combined with its high theoretical capacity (2980 mAh/g) and low cost, positions it as an attractive material for next-generation batteries, especially in large-scale applications like solar energy battery storage where cost and resource availability are critical factors.
The operational principle of aluminum-ion batteries revolves around the reversible electrochemical reaction of aluminum ions between the anode and cathode. Unlike lithium-ion batteries, which typically use graphite anodes, aluminum batteries utilize aluminum metal as the anode, which undergoes alloying reactions with other elements during discharge. This fundamental difference contributes to both the high energy potential and unique challenges of the technology.
One of the most significant breakthroughs in secondary aluminum-based battery technology came with the development of ionic liquid electrolytes, which have addressed many of the stability issues that plagued earlier designs. These electrolytes enable reversible aluminum plating and stripping, preventing the formation of passivation layers that previously hindered performance. The result is a battery system with excellent cycle stability, with some configurations demonstrating over 5,000 cycles with minimal capacity loss.
Cathode material development has been another area of intense research. Graphitic materials have shown particular promise, with their layered structure allowing for efficient intercalation of chloroaluminate anions. Recent innovations have yielded cathodes with enhanced specific capacities exceeding 120 mAh/g, significantly improving the overall energy density of aluminum-ion batteries. Other cathode materials under investigation include metal oxides, sulfides, and organic compounds, each offering distinct performance characteristics.
The safety profile of aluminum-based batteries is noteworthy. They are inherently resistant to thermal runaway and do not pose the same fire risks as some lithium-ion configurations. This makes them particularly suitable for residential and commercial solar energy battery storage systems, where safety considerations are paramount. Additionally, aluminum batteries are not prone to dendrite formation, further enhancing their safety and cycle life.
In terms of performance metrics, aluminum-ion batteries offer rapid charging capabilities, with some prototypes achieving full charge in under 60 seconds. This fast-charging ability is a significant advantage for applications requiring quick energy replenishment, including grid storage systems that need to respond rapidly to fluctuations in renewable energy generation. While energy density (currently ranging from 100-200 Wh/kg) is lower than that of lithium-ion batteries, ongoing research is steadily closing this gap.
Commercialization efforts are gaining momentum, with companies like Phinergy and others developing aluminum-air battery systems for electric vehicles and stationary storage. These systems leverage aluminum's high energy density and the abundance of oxygen from the air, resulting in remarkable energy storage capabilities that could transform solar energy battery storage by providing extended backup power during periods of low sunlight.
Challenges remaining for secondary aluminum-based battery technology include improving energy density further, enhancing low-temperature performance, and developing more cost-effective electrolyte solutions. Researchers are also working to address the issue of aluminum corrosion in certain electrolyte environments, which can affect long-term stability.
With continued advancements, aluminum-based batteries are expected to carve out a significant niche in the energy storage market. Their combination of low cost, high safety, rapid charging, and material abundance makes them particularly well-suited for large-scale applications, including utility-scale solar energy battery storage installations. As research progresses, we can anticipate aluminum-ion technology playing an increasingly important role in the global transition to renewable energy sources.
Aluminum Battery Construction
Detailed view of an aluminum-ion battery cell showing the aluminum anode, graphitic cathode, and ionic liquid electrolyte configuration.
Key Advantages
Exceptional Material Abundance
Aluminum is the most abundant metal in Earth's crust, ensuring long-term supply.
Enhanced Safety Profile
Resistant to thermal runaway and dendrite formation, minimizing fire risks.
Rapid Charging Capability
Can achieve full charge in minutes, or even seconds in optimized configurations.
Low Production Cost
Raw materials and manufacturing processes offer significant cost advantages.
Long Cycle Life
Demonstrates stable performance over thousands of charge-discharge cycles.
Secondary Zinc-Based Battery Technology
Secondary zinc-based battery technology has a long history of application in primary (non-rechargeable) configurations, but recent advancements have transformed it into a viable and compelling option for rechargeable energy storage systems. Zinc's unique combination of high energy density, low cost, inherent safety, and environmental friendliness has positioned it as a strong candidate for various applications, from consumer electronics to large-scale solar energy battery storage installations.
The fundamental operation of rechargeable zinc batteries involves the reversible plating and stripping of zinc metal at the anode during charge and discharge cycles. Depending on the specific chemistry, zinc batteries can utilize various cathode materials and electrolytes, each offering distinct performance characteristics. Among the most promising configurations are zinc-ion, zinc-air, zinc-bromine, and zinc-manganese dioxide systems, each with unique advantages for different applications.
One of the most significant challenges historically facing secondary zinc-based battery technology has been the formation of dendrites and shape change during cycling, which can lead to short circuits and reduced cycle life. However, recent innovations in electrolyte formulations, including the use of gel polymers, additives, and modified aqueous solutions, have significantly mitigated these issues. These advancements have enabled zinc batteries to achieve thousands of stable cycles, making them suitable for long-term energy storage applications.
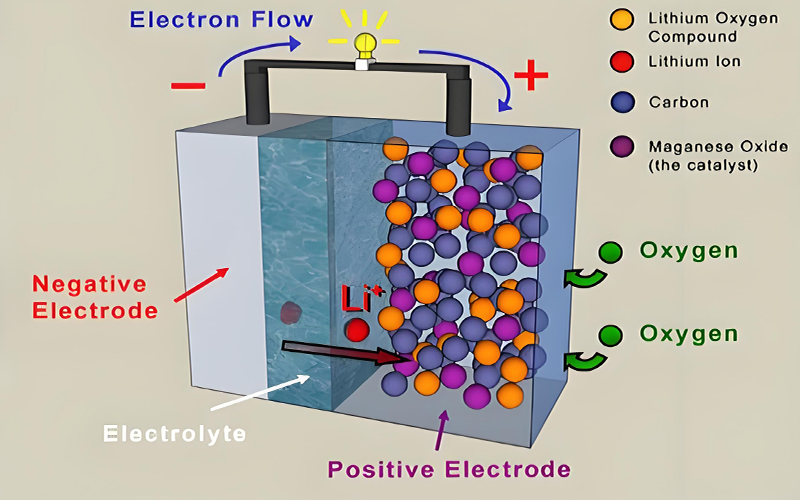
Zinc-air batteries represent a particularly exciting subset of this technology, offering exceptional energy density by utilizing oxygen from the atmosphere as the cathode reactant. This configuration eliminates the need for heavy metal cathodes, resulting in lightweight, high-capacity batteries with theoretical energy densities exceeding 1000 Wh/kg. While traditional zinc-air batteries suffered from limited cycle life, recent developments in bifunctional catalysts and membrane technologies have enabled rechargeable versions with significantly improved durability, making them attractive for solar energy battery storage applications where high energy density is crucial.
Another promising variant is the zinc-bromine flow battery, which has already seen commercial deployment in stationary energy storage systems. These flow batteries offer excellent scalability, long cycle life (exceeding 10,000 cycles), and low cost, making them well-suited for utility-scale applications. Their modular design allows for easy capacity expansion, and they demonstrate excellent performance in both deep discharge and partial state-of-charge scenarios typical in renewable energy integration.
The safety characteristics of secondary zinc-based battery technology are particularly noteworthy. Unlike some lithium-ion chemistries, zinc batteries are inherently non-flammable, even under extreme conditions. They operate in aqueous environments, eliminating the need for toxic or flammable organic electrolytes. This safety profile makes them ideal for residential solar energy battery storage systems, where proximity to living spaces demands the highest safety standards.
In terms of environmental impact, zinc batteries offer significant advantages. Zinc is abundant, easily recyclable, and poses minimal environmental hazards compared to other battery metals. The manufacturing process consumes less energy than lithium-ion battery production, and end-of-life recycling is more straightforward and cost-effective. These factors contribute to a significantly lower carbon footprint throughout the entire lifecycle, aligning well with the sustainability goals of renewable energy systems.
Commercial adoption of rechargeable zinc batteries is accelerating, with companies like Zinc8 Energy Solutions, Eos Energy Enterprises, and EnZinc bringing various technologies to market. These systems are being deployed in applications ranging from residential solar energy battery storage to utility-scale installations, demonstrating their versatility and performance capabilities.
Ongoing research in secondary zinc-based battery technology focuses on further improving cycle life, enhancing charge-discharge rates, and developing more efficient electrode materials. Innovations in nanostructured zinc electrodes, advanced membrane technologies, and novel electrolyte formulations continue to push performance boundaries. As these advancements continue, zinc-based batteries are expected to capture a significant share of the energy storage market, particularly in applications where safety, cost, and sustainability are paramount concerns.
Zinc-Air Battery System
Modular zinc-air battery configuration demonstrating the air cathode design that enables high energy density through oxygen utilization.
Performance Comparison
Zinc Battery Applications
Residential Storage
Home solar energy battery storage systems
Commercial Storage
Business and industrial energy systems
Grid Stabilization
Frequency regulation and load balancing
Transportation
Electric vehicles and marine applications
Secondary Calcium-Based Battery Technology
Secondary calcium-based battery technology represents one of the most innovative and emerging frontiers in energy storage research, offering unique properties that could potentially outperform many existing battery chemistries. As the fifth most abundant element in the Earth's crust, calcium provides a sustainable and cost-effective foundation for battery systems, with particular promise for large-scale applications such as solar energy battery storage where material availability and cost efficiency are critical factors.
The fundamental appeal of calcium-based batteries stems from calcium's high reduction potential (-2.87 V vs. SHE), which is very close to that of lithium (-3.04 V vs. SHE), suggesting the potential for high cell voltages and energy densities. Additionally, calcium ions carry a +2 charge, enabling higher charge transfer per ion compared to monovalent ions like lithium, which could translate to higher energy densities. Theoretical calculations suggest that calcium batteries could achieve energy densities exceeding 500 Wh/kg, making them competitive with the most advanced battery technologies currently under development.
Despite these promising characteristics, secondary calcium-based battery technology is still in its early stages of development, facing several significant technical challenges. The primary hurdle has been the development of suitable electrolytes that enable reversible calcium plating and stripping at the anode. Calcium's high reactivity with many common solvents and its tendency to form passivation layers on the electrode surface have historically hindered electrolyte development. However, recent breakthroughs using calcium bis(fluorosulfonyl)imide (Ca(FSI)₂) salts in ethereal solvents have shown promise in enabling reversible calcium electrochemistry.
Another major challenge in calcium battery research is the identification and development of cathode materials that can efficiently accommodate the relatively large calcium ions (100 pm ionic radius) during charge and discharge cycles. The larger ionic size creates significant diffusion limitations in many traditional electrode materials. Researchers have explored various material classes, including layered oxides, polyanionic compounds, and sulfides, with some promising results. Recent studies on titanium-based oxides and vanadium phosphates have demonstrated reversible calcium insertion, albeit at relatively low capacities.
Despite these challenges, progress in secondary calcium-based battery technology has accelerated in recent years. A significant breakthrough came in 2021 with the demonstration of a calcium-ion battery using a tin-based anode and a vanadium-based cathode, achieving a capacity of 60 mAh/g and stable cycling over 500 cycles. While these metrics are still modest compared to mature technologies, they represent an important proof of concept that has energized the research community.
One of the most exciting aspects of calcium-based batteries is their potential for integration with solar energy battery storage systems. Their projected high energy density would allow for compact storage solutions, while calcium's abundance ensures that large-scale production would not be limited by resource constraints. Additionally, calcium-based batteries are expected to have excellent safety profiles, as calcium is less reactive in air and water compared to lithium, reducing fire risks and simplifying handling and manufacturing processes.
Research efforts are currently focused on several key areas: developing more stable electrolytes with higher conductivity, identifying cathode materials with improved capacity and calcium ion mobility, and engineering electrode architectures that mitigate diffusion limitations. Nanostructuring electrode materials has shown particular promise in addressing diffusion challenges, with nanostructured cathodes demonstrating significantly improved rate capabilities compared to their bulk counterparts.
Another promising avenue of research is the development of calcium-air battery systems, which leverage oxygen from the atmosphere as the cathode reactant, similar to zinc-air batteries but with potentially higher energy densities. These systems could theoretically achieve energy densities approaching 2000 Wh/kg, which would be transformative for both mobile and stationary energy storage applications. While still in early stages, calcium-air research has demonstrated proof-of-concept devices with encouraging performance characteristics.
The environmental profile of secondary calcium-based battery technology is also highly favorable. Calcium compounds are generally non-toxic and abundant in nature, reducing environmental concerns throughout the battery lifecycle. Recycling processes for calcium batteries are expected to be simpler and more energy-efficient than for many other battery chemistries, aligning with the sustainability goals of renewable energy systems like solar energy battery storage.
While commercialization of calcium-based batteries is likely still 5-10 years away, the technology's potential has attracted significant research interest and investment. As understanding of calcium electrochemistry improves and new materials are developed, calcium-based batteries could emerge as a leading energy storage technology, particularly for applications requiring high energy density, low cost, and abundant materials. Their successful development would represent a major milestone in the advancement of sustainable energy storage solutions, contributing significantly to the global transition to renewable energy sources.
Calcium Battery Research
Laboratory research setup for developing calcium-based battery technologies, showing electrode preparation and testing equipment.
Research Progress Timeline
2015-2018
Early fundamental research on calcium electrochemistry and electrolyte development, identifying key challenges in reversible calcium plating.
2019-2020
Breakthrough electrolyte formulations enabling reversible calcium deposition and dissolution, overcoming passivation challenges.
2021-2022
First functional calcium-ion battery prototypes demonstrating reversible cycling with modest capacities and cycle life.
2023-Present
Significant improvements in cathode materials and cell design, with capacities exceeding 100 mAh/g and cycle life approaching 1000 cycles.
2025-2030 (Projected)
Expected commercialization of first generation calcium-based batteries for specialized applications, with potential for solar energy battery storage integration.
Technology Comparison
A comprehensive comparison of the four advanced battery technologies, highlighting their key performance metrics and suitability for various applications including solar energy battery storage.
| Performance Metric | Magnesium-Based | Aluminum-Based | Zinc-Based | Calcium-Based |
|---|---|---|---|---|
| Energy Density (Wh/kg) | 200-400 | 100-200 | 150-400 (up to 1000 for zinc-air) | 100-200 (projected 500+) |
| Cycle Life (cycles) | 1,000-5,000 | 2,000-10,000+ | 1,000-10,000+ | 500-1,000 (improving) |
| Charge Time | Moderate (1-4 hours) | Fast (minutes to 1 hour) | Moderate to slow (2-8 hours) | Moderate (1-5 hours) |
| Material Abundance | High (8th most abundant) | Very High (1st most abundant metal) | High (24th most abundant) | Very High (5th most abundant) |
| Cost Potential ($/kWh) | $100-200 | $50-150 | $50-100 | $75-150 (projected) |
| Safety Profile | Excellent (no dendrites) | Excellent (non-flammable) | Excellent (aqueous, non-toxic) | Very Good (low reactivity) |
| Commercial Readiness | Medium (5-7 years) | High (2-4 years) | Very High (1-3 years) | Low (7-10 years) |
| Solar Energy Battery Storage Suitability | Very High | High | Very High | High (future potential) |
Energy Density Comparison
Future Outlook
The ongoing development of these advanced battery technologies promises to revolutionize energy storage, enabling more efficient, sustainable, and accessible energy systems worldwide.
Continuous Innovation
Research into secondary magnesium-based battery technology, secondary aluminum-based battery technology, secondary zinc-based battery technology, and secondary calcium-based battery technology will accelerate, driven by the growing demand for efficient solar energy battery storage and electric transportation solutions.
Commercialization Milestones
Zinc-based technologies will lead commercialization in the next 3-5 years, followed by aluminum and magnesium systems. Calcium-based batteries will likely see initial commercial applications in specialized markets by 2030, with increasing relevance for solar energy battery storage.
Energy Transition Impact
These technologies will play a critical role in enabling the global transition to renewable energy by providing cost-effective, high-performance storage solutions. Their integration with solar energy battery storage systems will significantly reduce reliance on fossil fuels.
Ready to Power the Future?
Stay informed about the latest advancements in advanced battery technologies and their applications in solar energy battery storage systems.