1. Energy Conversion, Storage and Utilization
1.1 Energy Overview
Energy is the fundamental driving force behind modern civilization, enabling industrial processes, transportation, communication, and the basic comforts of daily life. The global energy landscape comprises various sources, including fossil fuels (coal, oil, natural gas), nuclear energy, and renewable resources (solar, wind, hydro, geothermal, biomass). Each energy source has distinct characteristics in terms of availability, environmental impact, conversion efficiency, and economic viability.
The transition from fossil fuels to renewable energy sources represents one of the most significant challenges of the 21st century. This shift is driven by concerns about climate change, energy security, and the finite nature of fossil fuel reserves. However, renewable energy sources are inherently intermittent and variable, creating a critical need for effective energy storage solutions. Battery storage systems have emerged as a key technology in addressing this challenge, enabling the integration of renewable energy into the grid by storing excess energy during periods of high generation and releasing it during periods of high demand.
Energy exists in various forms, including thermal, mechanical, electrical, chemical, and radiant energy. The ability to convert between these forms efficiently is essential for modern energy systems. For example, solar panels convert radiant energy into electrical energy, while internal combustion engines convert chemical energy into mechanical energy. Battery storage systems specialize in converting electrical energy into chemical energy for storage and back into electrical energy when needed.
The concept of energy efficiency has become increasingly important as global energy demand continues to rise. Efficient energy conversion and utilization minimize waste and reduce the overall environmental impact of energy systems. Battery storage plays a crucial role in improving energy efficiency by optimizing the match between energy supply and demand, reducing transmission losses, and enabling the recovery of energy that would otherwise be wasted.
1.2 Energy Storage and Energy Storage Technologies
Energy storage refers to the process of capturing energy produced at one time for use at a later time. This capability addresses the fundamental mismatch between energy supply and demand that exists in all energy systems. Energy storage technologies enable load leveling, peak shaving, frequency regulation, and backup power, among other applications.
The importance of energy storage has grown significantly with the increasing penetration of renewable energy sources. Unlike traditional fossil fuel power plants, which can be dispatched as needed, renewable energy generation depends on weather conditions and time of day. Battery storage systems provide a flexible solution to this variability, storing excess energy during periods of high renewable generation and releasing it when generation is low.

Figure 1: Integration of renewable energy sources with battery storage systems
Energy storage technologies can be categorized based on their energy storage mechanism, response time, storage capacity, and discharge duration. Each technology has its own set of advantages and limitations, making it suitable for specific applications. Battery storage, as one of the most versatile energy storage technologies, offers rapid response times, scalable capacity, and flexible deployment options, making it suitable for a wide range of applications from small-scale consumer electronics to utility-scale grid storage.
The development of energy storage technologies has accelerated in recent years, driven by declining costs, technological advancements, and supportive policy frameworks. Battery storage, in particular, has seen remarkable progress, with improvements in energy density, cycle life, and safety, along with significant cost reductions. These advancements have expanded the range of viable applications for battery storage, from electric vehicles to grid-scale energy management.
The integration of energy storage into energy systems offers numerous benefits, including increased renewable energy penetration, improved grid stability and reliability, enhanced energy security, and reduced greenhouse gas emissions. As the global energy transition continues, battery storage is expected to play an increasingly central role in enabling a more sustainable, resilient, and efficient energy future.
2. Classification and Applications of Energy Storage Technologies
Energy storage technologies encompass a diverse range of systems, each with unique characteristics and applications. These technologies can be broadly classified into several categories based on their energy storage mechanisms. The classification helps in understanding the suitability of each technology for specific applications based on factors such as power capacity, energy density, response time, cycle life, and cost.
2.1 Mechanical Energy Storage Technologies
Mechanical energy storage technologies convert electrical energy into mechanical energy for storage and back into electrical energy when needed. These systems typically offer large storage capacities and long lifespans, making them suitable for grid-scale applications.
Pumped Hydroelectric Storage (PHS) is the most mature and widely deployed mechanical energy storage technology. PHS systems use excess electricity to pump water from a lower reservoir to a higher elevation. When electricity is needed, the water is released back to the lower reservoir, driving turbines to generate electricity. PHS offers high efficiency (70-85%), long discharge durations, and decades of operational life, but requires specific geographical features and has high upfront costs.
Compressed Air Energy Storage (CAES) systems store energy by compressing air and storing it in underground caverns or pressure vessels. When electricity is needed, the compressed air is released, heated, and expanded through turbines to generate power. CAES offers large storage capacities but typically has lower efficiency (60-70%) compared to PHS.
Flywheel Energy Storage systems store energy in the form of rotational kinetic energy. A rotating mass (flywheel) is accelerated using an electric motor, storing energy. When needed, the flywheel's motion drives a generator to produce electricity. Flywheels offer very fast response times, high efficiency, and long cycle lives, but have relatively low energy density and require continuous power input to overcome friction losses. They are often used for short-duration energy storage and frequency regulation, complementing battery storage systems in hybrid applications.
2.2 Electrochemical Energy Storage Technologies
Electrochemical energy storage technologies, primarily battery storage systems, store energy through chemical reactions. These systems convert electrical energy into chemical energy during charging and reverse the process during discharge, producing electricity. Battery storage technologies offer high energy density, flexible scaling, and rapid response times, making them suitable for a wide range of applications from portable electronics to grid-scale storage.
Lead-acid batteries were the first commercially successful rechargeable battery technology and remain widely used in applications such as automotive starting, lighting, and ignition systems, as well as backup power. While relatively low in energy density and limited in cycle life compared to newer technologies, lead-acid batteries offer low cost and high reliability.
Lithium-ion batteries have emerged as the dominant battery storage technology for portable electronics, electric vehicles, and increasingly for stationary energy storage. These batteries offer high energy density, high efficiency (85-95%), and long cycle lives. Ongoing research and development continue to improve lithium-ion battery performance while reducing costs, further expanding their applications in battery storage systems.
Other electrochemical storage technologies include nickel-cadmium (NiCd), nickel-metal hydride (NiMH), sodium-sulfur (NaS), and flow batteries. Each technology has specific characteristics that make it suitable for particular applications. Flow batteries, for example, offer the advantage of decoupled power and energy capacity, making them well-suited for long-duration energy storage applications where battery storage systems need to deliver power over extended periods.
Comparison of Major Battery Storage Technologies
Figure 2: Performance comparison of different battery storage technologies
2.3 Electrical Energy Storage Technologies
Electrical energy storage technologies store energy in electrical fields or magnetic fields. These systems offer extremely fast response times, making them suitable for applications requiring rapid power delivery or absorption.
Capacitors and supercapacitors (ultracapacitors) store energy in an electric field. Supercapacitors can store significantly more energy than conventional capacitors and offer very high power density, extremely fast charge and discharge rates, and virtually unlimited cycle life. They are used in applications requiring short bursts of power, such as regenerative braking systems and power quality management, often in combination with battery storage systems to optimize overall performance.
Superconducting Magnetic Energy Storage (SMES) systems store energy in a magnetic field created by the flow of direct current in a superconducting coil. SMES systems offer very high efficiency (95% or higher), extremely fast response times (milliseconds), and high power density. However, they require cryogenic cooling to maintain superconductivity, which adds complexity and cost. SMES systems are used primarily for power quality applications, grid stabilization, and providing short-duration backup power.
2.4 Chemical Energy Storage Technologies
Chemical energy storage technologies convert electrical energy into fuels or other chemical compounds that can be stored and later converted back into electricity or used directly as fuels. These technologies, often referred to as power-to-X, offer the advantage of leveraging existing fuel infrastructure for storage and transportation.
Hydrogen energy storage involves using electricity to split water into hydrogen and oxygen through electrolysis. The hydrogen can be stored and later used in fuel cells to generate electricity, or used as a fuel in industrial processes, transportation, or heating. Hydrogen storage offers high energy density and long storage duration, but the overall efficiency of the process is relatively low (30-40%) compared to battery storage systems.
Other chemical storage technologies include synthetic fuels (such as methane, methanol, or ammonia) produced using renewable electricity. These fuels can be stored and transported using existing infrastructure and converted back to electricity when needed, or used directly in various applications. While these technologies offer long-term energy storage capabilities, they typically have lower round-trip efficiencies compared to battery storage systems, making them more suitable for seasonal storage or specific transportation applications.
2.5 Thermal Energy Storage Technologies
Thermal energy storage (TES) technologies store energy in the form of heat or cold, which can be used directly for heating or cooling applications or converted back to electricity. TES systems are particularly well-suited for integrating with renewable energy sources such as solar thermal power plants and improving the efficiency of industrial processes.
Sensible heat storage systems store energy by heating or cooling a material (such as water, rocks, or molten salts) without changing its phase. Molten salt storage, for example, is widely used in concentrated solar power plants, where heat from the sun is stored in molten salts and used to generate electricity when sunlight is unavailable.
Latent heat storage systems store energy through phase change materials (PCMs) that absorb or release heat when changing from solid to liquid or vice versa. PCMs offer high energy density and can maintain a constant temperature during phase change, making them suitable for space heating and cooling applications.
Thermochemical energy storage systems store energy through reversible chemical reactions. These systems can achieve very high energy densities and can store energy for long periods without significant losses. While still in the development stage for large-scale applications, thermochemical storage has the potential to complement battery storage systems in certain high-temperature industrial processes and renewable energy integration scenarios.
3. Overview of Storage Batteries
Storage batteries, often simply referred to as batteries, are devices that convert chemical energy into electrical energy through electrochemical reactions. Unlike primary batteries, which are disposable and cannot be recharged, storage batteries (also called secondary batteries) are designed to be recharged multiple times, making them essential components in countless applications requiring portable or backup power. The development of efficient, reliable battery storage systems has been instrumental in the advancement of modern technology, enabling everything from mobile communications to electric transportation and renewable energy integration.
The history of battery storage dates back to the late 18th century with Alessandro Volta's invention of the first electrochemical cell. Since then, battery technology has evolved significantly, with major breakthroughs including the development of lead-acid batteries in the 1850s, nickel-cadmium batteries in the early 20th century, and lithium-ion batteries in the 1970s and 1990s. Each advancement has brought improvements in energy density, power density, cycle life, and safety, expanding the capabilities and applications of battery storage systems.

Figure 3: Various types of storage batteries for different applications
Today, battery storage technology is at the forefront of the global energy transition. As renewable energy sources like solar and wind continue to grow in penetration, battery storage systems provide the flexibility needed to manage their variable output. These systems can store excess energy generated during periods of high production and release it when production is low or demand is high, helping to maintain grid stability and maximize the utilization of renewable resources.
The applications of battery storage are diverse and continue to expand. In consumer electronics, small, lightweight batteries power smartphones, laptops, tablets, and wearable devices. In transportation, battery electric vehicles (BEVs) and hybrid electric vehicles (HEVs) are becoming increasingly common, with advancements in battery storage technology driving improvements in range and performance. In stationary applications, battery storage systems provide backup power for homes and businesses, support microgrid operations in remote areas, and offer grid services such as frequency regulation and peak shaving.
The battery storage market has experienced remarkable growth in recent years, driven by declining costs, technological advancements, and supportive policies. According to industry reports, the global battery storage market is projected to continue expanding rapidly, with installations growing from gigawatt to terawatt scale over the next decade. This growth is expected to be fueled by both stationary storage applications and the electrification of transportation.
Despite significant progress, challenges remain in battery storage technology. These include improving energy density, extending cycle life, enhancing safety, reducing costs further, and addressing environmental concerns related to raw material extraction and battery recycling. Ongoing research and development efforts are focused on next-generation battery chemistries, such as solid-state batteries, lithium-sulfur batteries, and sodium-ion batteries, which promise to overcome many of the limitations of current technologies.
As battery storage technology continues to evolve, its role in the global energy system will become increasingly central. From enabling the widespread adoption of renewable energy to powering the electrification of transportation and industry, battery storage is poised to be a key enabler of a more sustainable, efficient, and resilient energy future. The ongoing innovation in this field will continue to expand the possibilities for energy storage, opening up new applications and transforming how we generate, distribute, and consume energy.
4. Working Principles and Composition of Storage Batteries
4.1 Working Principles of Storage Batteries
Storage batteries operate based on reversible electrochemical reactions that convert between electrical energy and chemical energy. This reversibility is what allows battery storage systems to be charged and discharged multiple times. The basic unit of a battery is an electrochemical cell, which consists of two electrodes (a positive electrode or cathode and a negative electrode or anode) immersed in an electrolyte.
During discharge, chemical reactions occur at both electrodes, generating electrons that flow through an external circuit, creating an electric current. At the anode (negative electrode), oxidation takes place, releasing electrons. These electrons travel through the external circuit to the cathode (positive electrode), where reduction occurs, consuming electrons. The electrolyte facilitates the movement of ions between the electrodes to maintain charge neutrality within the cell.
During charging, an external power source applies a voltage greater than the cell's open-circuit voltage, reversing the electrochemical reactions. This forces electrons to flow in the opposite direction, converting electrical energy back into chemical energy stored in the electrodes and electrolyte. The ability to reverse these reactions efficiently and repeatedly is a key characteristic of effective battery storage systems.
The specific chemical reactions vary depending on the battery chemistry. For example, in lead-acid batteries, the discharge process involves the reaction of lead and lead dioxide with sulfuric acid to form lead sulfate and water. During charging, this reaction is reversed, regenerating lead, lead dioxide, and sulfuric acid. In lithium-ion batteries, the process involves the movement of lithium ions between electrodes, with electrons flowing through the external circuit to balance the charge.
The performance of battery storage systems is influenced by various factors related to their working principles, including the efficiency of the electrochemical reactions, the rate of ion diffusion in the electrolyte, the conductivity of the electrodes, and the stability of the materials involved. Understanding these principles is essential for optimizing battery design, operation, and performance in different applications.
4.2 Composition of Storage Batteries
While the specific materials vary depending on the battery chemistry, all storage batteries share a basic structure consisting of several key components working together to enable the electrochemical energy conversion process. These components include electrodes, electrolyte, separator, and casing, each playing a critical role in the performance and safety of the battery storage system.
Anatomical Structure of a Typical Storage Battery
-
1
Cathode (Positive Electrode): Typically made of metal oxides or sulfides that undergo reduction during discharge.
-
2
Anode (Negative Electrode): Usually composed of materials that oxidize easily, releasing electrons during discharge.
-
3
Electrolyte: Conductive medium that allows ion migration between electrodes while preventing direct electron flow.
-
4
Separator: Physical barrier between electrodes preventing short circuits while allowing ion transport.
-
5
Casing: Protective enclosure holding all components, often designed to prevent leakage and manage thermal conditions.
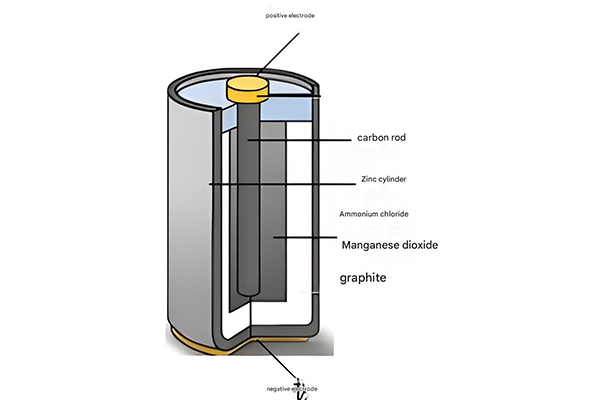
Figure 4: Main components of a typical storage battery
The electrodes are the heart of any battery storage system, where the electrochemical reactions take place. The cathode (positive electrode) and anode (negative electrode) are typically made of materials that can reversibly accept and release ions. In lithium-ion batteries, common cathode materials include lithium cobalt oxide, lithium iron phosphate, and lithium nickel manganese cobalt oxide, while anodes are often made of graphite or other carbon-based materials.
The electrolyte serves as the medium for ion transport between the electrodes. It can be a liquid, gel, or solid material with high ionic conductivity but low electronic conductivity to prevent self-discharge. Liquid electrolytes are common in many battery types, including lead-acid and conventional lithium-ion batteries, while solid-state electrolytes are an area of active research for next-generation battery storage systems.
The separator is a critical safety component that physically separates the cathode and anode to prevent short circuits while allowing ions to pass through. Separators are typically made of porous polymeric materials designed to maintain their structure under normal operating conditions and, in some cases, to shut down ion transport if the battery overheats, providing a safety mechanism.
Battery cells are often combined in series and parallel configurations to achieve the desired voltage and capacity for specific applications. These combinations, along with protective circuitry (Battery Management Systems or BMS), form complete battery storage packs. The BMS monitors and controls various aspects of battery operation, including cell balancing, temperature management, charge/discharge rates, and protection against overcharging, over-discharging, and short circuits.
The selection of materials for each component is critical to the performance, safety, cost, and environmental impact of battery storage systems. Researchers continue to develop new materials and designs to improve energy density, power density, cycle life, safety, and sustainability, driving the ongoing evolution of battery storage technology.
5. Performance Indicators and Related Terminology
Understanding the performance characteristics of battery storage systems is essential for selecting the right technology for a specific application, optimizing system design, and evaluating overall system efficiency. Various metrics and terminology are used to describe the performance, capabilities, and limitations of battery storage systems. These indicators help in comparing different technologies, predicting system behavior, and ensuring safe and efficient operation.
5.1 Electromotive Force (EMF)
Electromotive force (EMF) is the potential difference generated by a battery when no current is flowing through it. It represents the maximum possible voltage that a battery can deliver and is a measure of the driving force behind the electrochemical reactions within the cell. EMF is determined by the specific chemical reactions and materials used in the battery, remaining relatively constant for a given battery chemistry.
Unlike voltage, which decreases as current flows due to internal resistance, EMF remains constant under open-circuit conditions. It is typically measured in volts (V) and serves as a fundamental characteristic of a battery's chemical composition. For example, a lead-acid battery cell has an EMF of approximately 2.0 volts, while a lithium-ion battery cell typically has an EMF ranging from 3.2 to 3.7 volts, depending on the specific chemistry. Understanding the EMF of a battery storage system is essential for designing appropriate charging systems and predicting overall system voltage under various operating conditions.
5.2 Open Circuit Voltage (OCV)
Open Circuit Voltage (OCV) is the voltage measured across a battery's terminals when no load is connected, meaning no current is flowing. While often used interchangeably with EMF, OCV is technically the measurable voltage that approximates the EMF but may differ slightly due to various factors, including temperature, state of charge, and battery age.
OCV is a critical parameter in battery storage systems as it provides valuable information about the battery's state of charge (SOC). For many battery chemistries, there is a predictable relationship between OCV and SOC, allowing OCV measurements to be used as a simple method for estimating the remaining charge in a battery. This relationship is often represented in a discharge curve, which plots voltage against the depth of discharge.
Monitoring OCV is also important for assessing battery health and detecting potential issues. Significant deviations from the expected OCV for a given SOC can indicate problems such as cell imbalance, degradation, or internal short circuits. In battery management systems for sophisticated battery storage applications, OCV measurements are used to optimize charging profiles, prevent overcharging and over-discharging, and ensure safe operation.
5.3 Internal Resistance
Internal resistance is the opposition to the flow of current within a battery, caused by the resistance of the electrodes, electrolyte, and other internal components. It is measured in ohms (Ω) and plays a crucial role in determining the performance of battery storage systems, particularly their ability to deliver high currents.
When current flows through a battery, the internal resistance causes a voltage drop according to Ohm's law (V = IR), where V is the voltage drop, I is the current, and R is the internal resistance. This voltage drop reduces the effective voltage available at the battery terminals, especially under high load conditions. Batteries with lower internal resistance can deliver higher currents more efficiently and maintain a more stable voltage under varying load conditions.
Internal resistance is not a constant value but varies with factors such as state of charge, temperature, and battery age. It typically increases as the battery discharges and as it ages, which is one reason why older batteries often perform poorly compared to new ones. Cold temperatures also tend to increase internal resistance, which is why battery performance often decreases in cold environments.
Measuring internal resistance is an important method for assessing the health and performance of battery storage systems. A significant increase in internal resistance over time indicates battery degradation and can help predict when replacement may be necessary. For battery storage system designers, understanding internal resistance characteristics is essential for optimizing system performance, particularly for applications requiring high power output.
5.4 Operating Voltage
Operating voltage, also known as working voltage, is the actual voltage delivered by a battery when it is supplying current to a load. Unlike open circuit voltage, which is measured with no current flow, operating voltage decreases as current increases due to the battery's internal resistance.
The relationship between operating voltage, open circuit voltage, current, and internal resistance is described by the equation: V_operating = V_oc - I*R_internal, where V_operating is the operating voltage, V_oc is the open circuit voltage, I is the current, and R_internal is the internal resistance. This relationship explains why a battery's voltage drops under load, particularly high loads.
The operating voltage of a battery storage system changes throughout the discharge cycle, following a characteristic curve that depends on the battery chemistry, discharge rate, and temperature. This curve typically shows a relatively stable voltage during most of the discharge cycle, with more significant drops at the beginning and end. The minimum acceptable operating voltage is an important parameter, as discharging below this level can cause damage to the battery and reduce its lifespan.
For practical applications, battery storage systems are often designed with multiple cells connected in series to achieve the desired operating voltage. The operating voltage range is a key consideration in system design, as it must be compatible with the equipment or system being powered. Battery management systems in modern battery storage applications monitor operating voltage continuously to optimize performance, prevent damage, and ensure safe operation.
5.5 Discharge Performance
Discharge performance refers to how a battery delivers its stored energy under various conditions, including different discharge rates, temperatures, and states of health. It is a critical characteristic for determining the suitability of a battery storage system for specific applications, as different applications have varying requirements for power delivery and duration.
One key aspect of discharge performance is the capacity delivered at different discharge rates. Most battery storage systems can deliver their full rated capacity when discharged at a moderate rate, but capacity decreases at higher discharge rates. This phenomenon is often described using the C-rate, which is a measure of the discharge current relative to the battery's rated capacity. For example, a 1C discharge rate for a 100Ah battery would be 100A, which would theoretically fully discharge the battery in 1 hour. A 2C rate would be 200A, discharging the battery in approximately 30 minutes but with reduced total capacity.
Temperature significantly affects discharge performance, with most batteries delivering optimal performance within a moderate temperature range (typically 20-25°C). Cold temperatures increase internal resistance and reduce available capacity, while high temperatures can accelerate degradation and potentially compromise safety. Battery storage systems often include thermal management systems to maintain optimal operating temperatures, particularly in applications with high power demands or extreme environmental conditions.
The discharge profile, or the shape of the voltage curve during discharge, is another important aspect of discharge performance. Some battery chemistries maintain a relatively flat voltage profile throughout most of the discharge cycle, which is desirable for applications requiring stable voltage. Others exhibit more gradual voltage decline. The discharge profile affects how effectively the battery's capacity can be utilized in different applications.
Cycle life, the number of charge-discharge cycles a battery can undergo before its capacity degrades to a specified level (typically 80% of the original capacity), is closely related to discharge performance. Discharging to deeper levels (higher depth of discharge) generally reduces cycle life. Battery management systems optimize discharge performance by controlling the depth of discharge and preventing operation outside of safe parameters.
Understanding discharge performance is essential for selecting appropriate battery storage systems and designing efficient energy systems. For example, applications requiring short bursts of high power (like starting a vehicle) prioritize high C-rate performance, while applications requiring long-duration energy delivery (like off-grid solar systems) prioritize capacity retention at low discharge rates. Evaluating discharge performance under realistic operating conditions ensures that battery storage systems meet the specific requirements of their intended application.