Nickel-Metal Hydride Battery Technology
A comprehensive technical overview of one of the most reliable energy storage solutions in the modern energy storage system landscape
1. Construction, Working Principle and Performance of Nickel-Metal Hydride Batteries
1.1 Construction of Nickel-Metal Hydride Batteries
The nickel-metal hydride (Ni-MH) battery is a sophisticated electrochemical device composed of several key components working in harmony to deliver reliable energy storage. As a critical component in many energy storage system applications, its construction is carefully engineered to balance performance, safety, and longevity.
At the core of every Ni-MH battery are the two electrodes: the positive electrode (cathode) and the negative electrode (anode). The cathode typically consists of nickel hydroxide (Ni(OH)₂) mixed with small amounts of other elements such as cobalt, zinc, and manganese. These additives enhance conductivity and structural stability during charge-discharge cycles, making the battery more suitable for demanding energy storage system requirements.
The anode is composed of a hydrogen-absorbing alloy (metal hydride), which gives the battery its name. This alloy has the unique ability to absorb and release hydrogen ions during electrochemical reactions. Common alloys include AB₅-type (where A is a rare earth metal mixture and B is nickel, cobalt, manganese, or aluminum), AB₂-type (titanium-zirconium-based), and various other intermetallic compounds.
Separating the two electrodes is a porous separator, typically made from polypropylene or polyethylene. This separator allows the flow of ions while preventing physical contact between the electrodes that would cause a short circuit – a crucial safety feature in any energy storage system.
The electrolyte, a potassium hydroxide (KOH) solution in water, fills the space between the electrodes and separator, facilitating the movement of hydroxide ions (OH⁻) during charge and discharge cycles. The entire assembly is enclosed in a metal or plastic casing that protects the internal components and prevents electrolyte leakage.
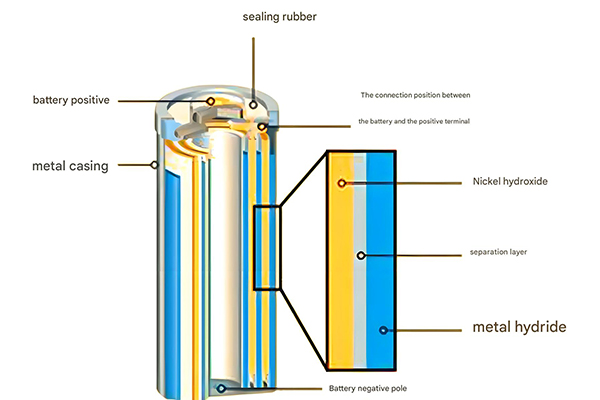
1.2 Working Principle of Nickel-Metal Hydride Batteries
The operation of a nickel-metal hydride battery is based on reversible electrochemical reactions that occur at both electrodes during charging and discharging. This reversibility is what makes Ni-MH technology so valuable in energy storage system applications where repeated charge-discharge cycles are required.
During the charging process, electrical energy is converted into chemical energy. At the cathode (positive electrode), nickel hydroxide (Ni(OH)₂) reacts with hydroxide ions (OH⁻) to form nickel oxyhydroxide (NiOOH), water (H₂O), and electrons (e⁻). This reaction can be represented as:
Ni(OH)₂ + OH⁻ → NiOOH + H₂O + e⁻
Simultaneously, at the anode (negative electrode), hydrogen ions (H⁺) – generated from the dissociation of water – combine with electrons and the metal alloy (M) to form a metal hydride (MH). This reaction is:
M + H₂O + e⁻ → MH + OH⁻
During discharge, these reactions reverse as chemical energy is converted back to electrical energy to power devices or feed into an energy storage system. Electrons flow from the anode to the cathode through an external circuit, creating an electric current. The hydroxide ions move through the electrolyte and separator to maintain charge neutrality.
This reversible process allows Ni-MH batteries to undergo hundreds, even thousands, of charge-discharge cycles, making them a durable choice for various energy storage system applications from portable electronics to hybrid vehicles.
Fig 1.2: Charge-Discharge Cycle in Ni-MH Batteries
1.3 Performance of Nickel-Metal Hydride Batteries
The performance characteristics of nickel-metal hydride batteries make them particularly suitable for specific energy storage system applications where a balance of energy density, power density, and cycle life is required. Understanding these performance metrics is crucial for selecting the right energy storage system technology for a given application.
Energy density – the amount of energy stored per unit volume or mass – typically ranges from 60-120 Wh/kg and 140-300 Wh/L for Ni-MH batteries. This places them between lead-acid batteries (lower energy density) and lithium-ion batteries (higher energy density), making them a mid-range option in the energy storage system spectrum.
Power density – the rate at which energy can be delivered – is generally 200-1000 W/kg, enabling Ni-MH batteries to provide high current outputs necessary for applications like electric vehicle acceleration. This power capability makes them valuable in energy storage system setups requiring rapid energy discharge.
Cycle life – the number of charge-discharge cycles before capacity drops to 80% of original – typically ranges from 500-1000 cycles under normal conditions, though some advanced designs can achieve 2000+ cycles. This longevity is a key advantage in energy storage system applications where replacement costs are high.
Operating temperature range is another important performance parameter, with Ni-MH batteries typically functioning reliably between -20°C and 60°C, though performance may degrade at extreme temperatures. This makes them suitable for energy storage system deployment in various climatic conditions.
Self-discharge rate is relatively high compared to some other battery technologies, ranging from 20-30% per month, which can be a consideration in energy storage system applications requiring long periods of standby. However, recent advancements have reduced this rate significantly in premium Ni-MH formulations.
Fig 1.3: Performance Comparison of Battery Technologies
2. Key Materials and Technologies in Nickel-Metal Hydride Batteries
2.1 Classification and Properties of Hydrogen Storage Alloys
Hydrogen storage alloys are the critical materials that enable the functionality of nickel-metal hydride batteries, serving as the anode where hydrogen is stored during charging. These alloys' unique properties directly influence the performance of the entire energy storage system.
The most common classification is based on their chemical composition and crystal structure:
AB₅-type alloys
These are mischmetal (Mm) based alloys with a hexagonal CaCu₅-type structure, where A represents a rare earth metal mixture (lanthanum, cerium, neodymium, praseodymium) and B is a combination of nickel, cobalt, manganese, and aluminum (MmNi₅₋ₓ-y-zCoxMnyAlz). They offer excellent activation characteristics, good high-rate discharge performance, and relatively low cost, making them popular in consumer energy storage system applications.
AB₂-type alloys
These titanium-zirconium-based alloys (e.g., Ti-Zr-Ni-V) with a Laves phase structure have higher hydrogen storage capacity (1.8-2.0 wt% vs. 1.3-1.4 wt% for AB₅) and better cycle life. Their higher energy density makes them attractive for energy storage system applications requiring extended runtime, though they are more expensive and require more complex activation procedures.
Other types
These include A₂B-type (Mg-based), AB-type (TiFe), and V-based solid solution alloys. Magnesium-based alloys offer very high theoretical capacity but suffer from poor kinetics and cycle life. Vanadium-based alloys provide excellent hydrogen storage properties but are costly, limiting their use in commercial energy storage system applications.
| Alloy Type | Capacity (mAh/g) | Cycle Life | Cost |
|---|---|---|---|
| AB₅-type | 280-330 | 500-1000 | Low |
| AB₂-type | 350-450 | 1000-2000 | Medium |
| Mg-based | 500-1000 | 100-300 | Low |
| V-based | 380-500 | 800-1500 | High |
Table 2.1: Properties of Different Hydrogen Storage Alloys
2.2 Preparation of Hydrogen Storage Alloys
The preparation method of hydrogen storage alloys significantly impacts their microstructure, hydrogen storage properties, and overall performance in a nickel-metal hydride battery. For commercial energy storage system production, consistent alloy quality and performance are paramount.
Induction melting
This is the most common industrial method, where raw materials are melted in a crucible using induction heating under an inert atmosphere (argon) to prevent oxidation. The molten alloy is then cast into molds to form ingots. This method allows precise control over composition and produces homogeneous alloys suitable for large-scale energy storage system manufacturing.
Arc melting
Used primarily for laboratory-scale production, this method involves melting materials using an electric arc between a tungsten electrode and the metal charge in a water-cooled copper crucible. Multiple meltings are performed to ensure homogeneity. While not ideal for mass production, arc melting is valuable for developing new alloy compositions for advanced energy storage system applications.
Rapid solidification
Techniques such as melt spinning produce alloys with fine, homogeneous microstructures by rapidly cooling molten metal on a rotating copper wheel (cooling rates of 10⁵-10⁶ K/s). This results in improved cycle life and high-rate discharge performance compared to conventionally cast alloys, making them suitable for high-performance energy storage system applications.
Mechanical alloying
This powder metallurgy technique involves high-energy ball milling of elemental powders to create alloy powders through solid-state reactions. It can produce metastable phases and alloys that are difficult to prepare by melting. Mechanical alloying is particularly useful for manufacturing magnesium-based alloys and offers advantages in energy storage system applications requiring specific powder characteristics.
Typical Alloy Preparation Process
- Raw material selection and purification
- Melting under inert atmosphere
- Casting and solidification
- Homogenization heat treatment
- Crushing and grinding to powder
- Surface treatment
- Quality control and testing
Fig 2.2: Hydrogen Storage Alloy Manufacturing Process
2.3 Performance Degradation and Surface Treatment of Hydrogen Storage Alloys
Performance degradation of hydrogen storage alloys over repeated charge-discharge cycles is a critical issue affecting the longevity and reliability of nickel-metal hydride batteries in energy storage system applications. Understanding these degradation mechanisms and implementing effective surface treatments are key to improving energy storage system durability.
Degradation mechanisms
Volume expansion/contraction: During hydrogen absorption and desorption, alloys can undergo volume changes of 15-25%, leading to microcracking, powdering, and loss of electrical contact in the electrode. This is the primary degradation mechanism in most energy storage system applications utilizing Ni-MH technology.
Oxidation/corrosion: Exposure to the alkaline electrolyte can cause surface oxidation of the alloy, forming a passive layer that hinders hydrogen diffusion and electron transfer. This is particularly problematic in energy storage system applications operating at elevated temperatures.
Alloy composition changes: Selective leaching of certain elements (e.g., aluminum, manganese) into the electrolyte can alter the alloy's crystalline structure and hydrogen storage properties over time.
Surface treatment technologies
Chemical treatment: Immersion in acids, alkalis, or complexing agents removes surface oxides and creates a porous structure that enhances hydrogen diffusion. This cost-effective treatment is widely used in energy storage system manufacturing.
Electroless plating: Depositing thin layers of metals (Ni, Co, Cu) on the alloy surface improves conductivity, protects against corrosion, and reduces粉化. This treatment significantly extends cycle life in demanding energy storage system applications.
Ion sputtering and coating: Physical vapor deposition techniques apply thin, uniform coatings that provide a barrier against electrolyte attack while maintaining hydrogen permeability. These advanced treatments are used in high-performance energy storage system designs.
Effect of Surface Treatments on Cycle Life
Fig 2.3: Alloy Degradation and Surface Treatment Effects
2.4 Auxiliary Materials for Nickel-Metal Hydride Batteries
While the electrodes and hydrogen storage alloys are the active components of nickel-metal hydride batteries, several auxiliary materials play crucial roles in ensuring optimal performance, safety, and manufacturability. These materials are essential for integrating Ni-MH technology into efficient energy storage system solutions.
Separators
Typically made from polypropylene or polyethylene non-woven fabrics, separators must provide high ionic conductivity, good mechanical strength, and chemical stability in alkaline electrolyte. Modern separators for advanced energy storage system applications feature optimized porosity (40-60%) and thickness (20-50 μm) to balance ion transport and mechanical integrity.
Electrolytes
Aqueous potassium hydroxide (KOH) solutions (6-8 M) are standard, sometimes mixed with LiOH and NaOH to improve performance. The electrolyte facilitates ion transport between electrodes and must remain stable throughout the battery's operating life – a critical factor in energy storage system reliability.
Binder materials
These materials hold electrode active materials together and adhere them to current collectors. For cathodes, polyvinyl alcohol (PVA) or carboxymethyl cellulose (CMC) mixed with polytetrafluoroethylene (PTFE) are common. Anodes typically use PTFE or styrene-butadiene rubber (SBR) binders that maintain stability in the alkaline environment. Binder selection directly impacts electrode conductivity and mechanical strength in energy storage system applications.
Current collectors
These are typically made from nickel – expanded metal, foam, or mesh for cathodes and thin nickel sheets or meshes for anodes. Their design maximizes surface area while minimizing electrical resistance, crucial for high-rate performance in energy storage system applications requiring rapid charge and discharge.
Additives
Various additives improve specific properties: surfactants enhance electrolyte wetting, corrosion inhibitors protect current collectors, and organic compounds can reduce self-discharge. These additives are carefully formulated to address specific energy storage system application requirements, from low-temperature performance to extended shelf life.

Separators
Ion transport & electrical isolation
Electrolytes
Ionic conduction medium
Binders
Electrode structural integrity
Current Collectors
Electron conduction pathway
Fig 2.4: Auxiliary Materials in Ni-MH Batteries
Technical Summary
Nickel-metal hydride battery technology continues to be a valuable component in the energy storage system landscape, offering a balance of performance, safety, and cost-effectiveness. Its unique construction, relying on hydrogen storage alloys and carefully engineered auxiliary materials, enables reliable operation across numerous applications. While facing competition from lithium-ion technologies, Ni-MH batteries maintain advantages in specific energy storage system applications requiring robust performance, wide operating temperature ranges, and enhanced safety profiles. Ongoing research into advanced alloys, improved manufacturing processes, and innovative surface treatments continues to extend the capabilities of Ni-MH technology, ensuring its relevance in the evolving energy storage system market.